Comprehensive explanation of lithium-ion battery knowledge-Tritek Battery R&D Director
With the development of the global new energy industry, lithium batteries have become a high-demand industry, and power lithium batteries have become a concentrated area of demand growth for the lithium battery industry.
So do you fully understand the technical knowledge of lithium-ion batteries? Here’s a comprehensive guide summarizing it from Tritek’s R&D Director.
We have also prepared a glossary of electric bicycle terms (https://tritekbattery.com/zh-CN/ebike-glossary-of-terms/) for your quick search.
Content [hide]
1 Basic knowledge of cells
1.1 History of battery development
1.2 Battery category
1.3 Development direction of power batteries
1.4 Lithium battery – development history
1.5 Lithium Battery – How It Works
1.6 Lithium batteries – working principle – charging and discharging processes
1.7 Lithium battery-structural composition
1.8 Lithium battery – cathode material
1.9 Lithium Batteries – Classification
1.10 Lithium battery-performance parameters
1.11 1.4 Lithium battery – charge and discharge curve
1.12 Lithium battery-high and low temperature performance
1.13 Lithium battery-high and low temperature performance
1.14 Lithium battery – cycle performance at different charging rates
1.15 Lithium battery – cycle performance at different discharge rates
1.16 Lithium Battery – Effect of DOD on Cycle Life
1.17 Lithium Battery – Effect of Temperature on Cycle Life
1.18 Lithium battery-normal temperature rate discharge, discharge efficiency, temperature rise
1.19 Lithium battery – normal temperature rate charging, charging efficiency, temperature rise
1.20 Lithium battery – principle of doubled charge and discharge temperature rise
1.21 Lithium battery-matching battery PACK
2 Cell material knowledge
2.1 Knowledge of battery core materials – positive electrode
2.2 Knowledge of battery core materials – negative electrode
2.3 Knowledge of battery core materials – conductive agent
2.4 Battery material knowledge—binders
2.5 Knowledge of battery core materials – foil
2.6 Battery core material knowledge——Lug
2.7 Battery core material knowledge – separator
2.8 Battery material knowledge – electrolyte
2.9 Knowledge of battery core materials – steel shell
2.10 Battery material knowledge——Cap
2.11 Battery core material knowledge – safety device
3 Cell technology knowledge
3.1 Cell process knowledge-process flow
4. Cell structure knowledge
4.1 Knowledge of cell structure
4.2 Knowledge of battery core structure – square stack
4.3 Knowledge of battery core structure – square Z-shaped lamination
4.4 Knowledge of battery core structure – square film lamination
4.5 Knowledge of battery core structure – square multi-lug winding
4.6 Knowledge of battery core structure – cylindrical single multi-lug winding
4.7 Knowledge of battery core structure – cylindrical full lug winding
4 8 Cell Structure Knowledge-Summary
Cell basics
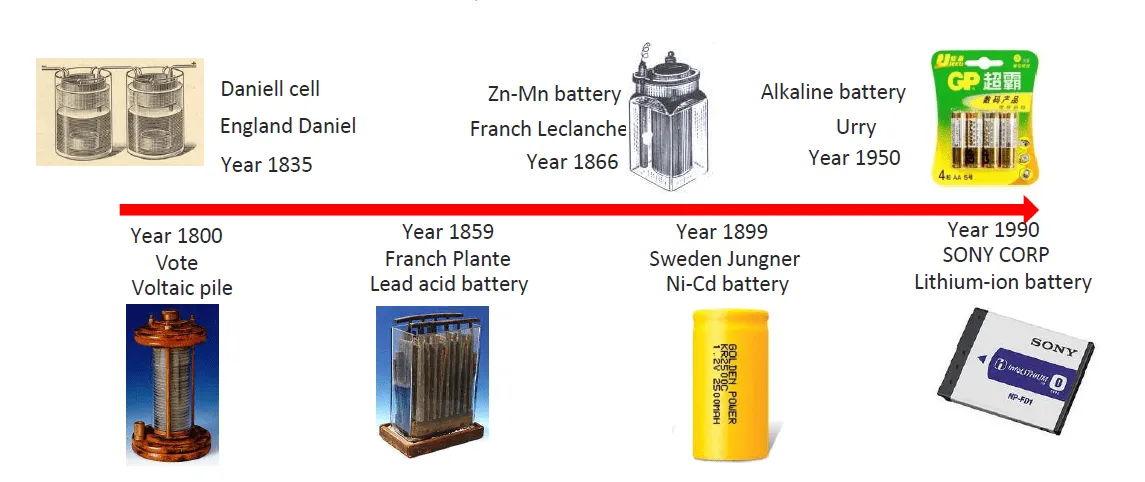
battery development
A battery is a device that converts chemical energy into electrical energy. It directly provides electrical energy to the outside world through chemical reactions within the battery.
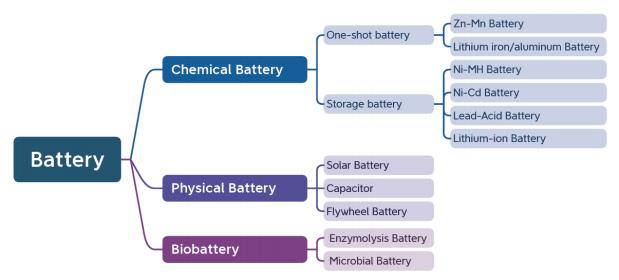
Battery category
Batteries are mainly divided into three types: chemical batteries, physical batteries and biological batteries. Below is a mind map of battery classification.
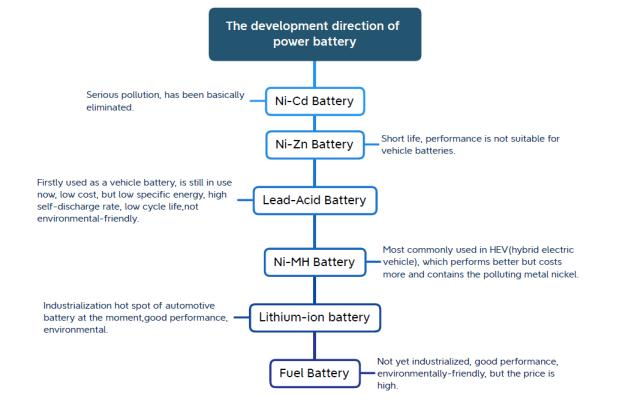
The development direction of power batteries
Nickel-cadmium battery → Nickel-zinc battery → Lead-acid battery → Ni-MH battery → Lithium-ion battery → Fuel cell
Nickel-cadmium batteries: seriously polluted and have been basically eliminated.
Nickel-zinc battery: short life, performance is not suitable for car batteries.
Lead-acid battery: It was first used as a vehicle battery and is still in use today. It has low cost, but low specific energy, high self-discharge rate, low cycle life, and is not environmentally friendly.
Nickel Metal Hydride Batteries: Most commonly used in HEVs (Hybrid Electric Vehicles), perform better but cost more and contain the polluting metal nickel.
Lithium-ion battery: The current hot spot in the automotive battery industry, with good performance and environmental protection.
Triteks’ LEV products such as e-bike batteries, custom e-bike batteries, electric motorcycle batteries, cargo bike batteries all use lithium-ion batteries.
Fuel cell: Not yet industrialized, has good performance and is environmentally friendly, but the price is high.
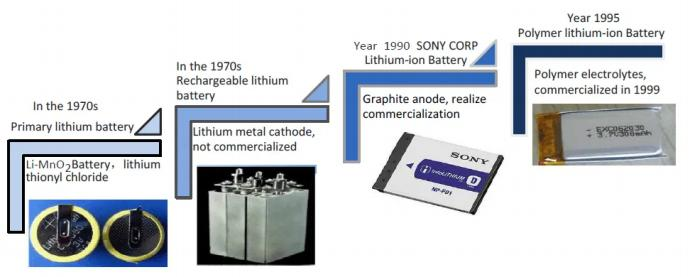
Lithium battery – development history
Carbon is used as the negative electrode and lithium compound is used as the positive electrode; during the charge and discharge process, lithium ions shuttle back and forth between the positive electrode and the negative electrode, hence the name of the lithium-ion battery.
Lithium Battery – How It Works
During the charging and discharging process of lithium-ion batteries, lithium ions are in a state of movement from the positive electrode to the negative electrode and then to the positive electrode. It’s like a rocking chair, with the lithium ions moving back and forth between the two ends of the battery. This electrochemical energy storage system is called a “rocking chair battery.”
Lithium batteries – working principle – charging and discharging processes
1.6.1 Lithium ions are embedded in the layered structure of the cathode material before charging.
1.6.2 After charging starts, the positive electrode material loses electrons and lithium ions escape from the positive electrode material.
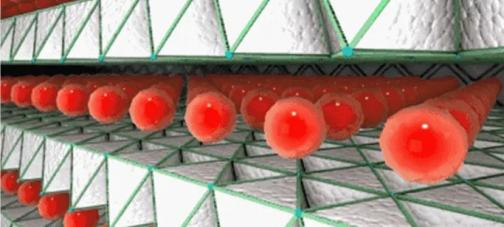
1.6.3 Lithium ions reach the negative electrode graphite material through the electrolyte and separator.

1.6.4 Lithium ions are embedded in the graphite layer, while electrons reach the negative electrode through the external circuit, forming relatively stable lithium-embedded graphite.
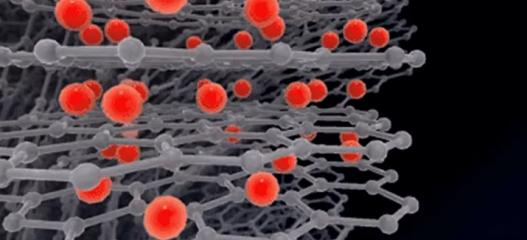
1.6.5 As the charging process continues, the positive electrode material continues to lose electrons and lithium ions continue to be deintercalated until the charging is completed.
1.6.6 Electrons leave the negative electrode material and flow to the positive electrode through the external circuit. Lithium ions that have lost electrons will also escape from the graphite layer.
1.6.7 The lithium ions extracted from the negative electrode return to the positive electrode material through the electrolyte and separator, and combine with the electrons reaching the positive electrode through the external circuit to form a relatively stable lithium-embedded positive electrode material.
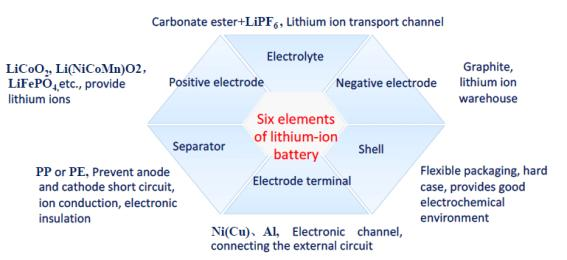
Lithium battery-structural composition
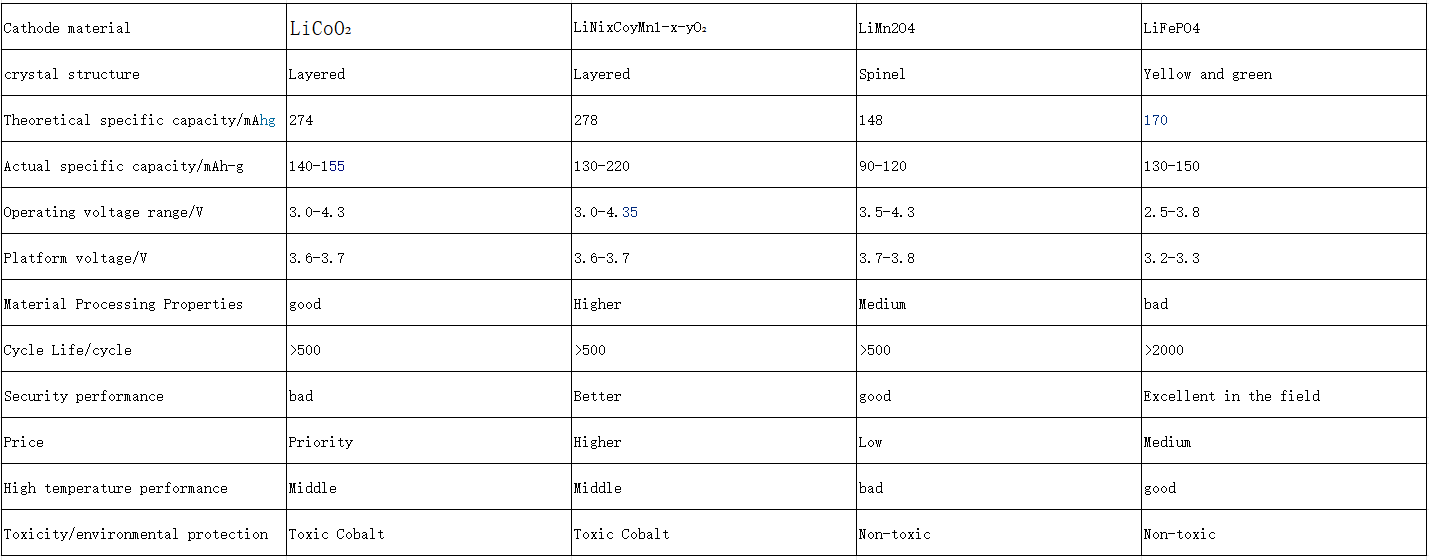
Lithium battery – cathode material
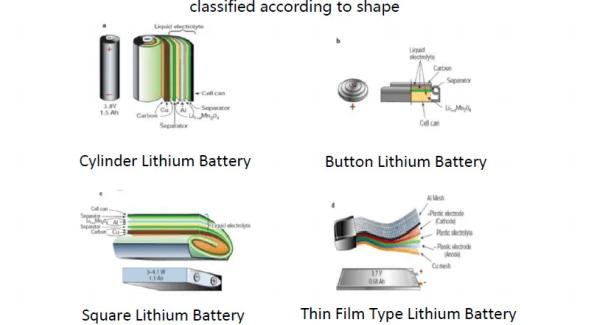
Lithium Batteries – Classification
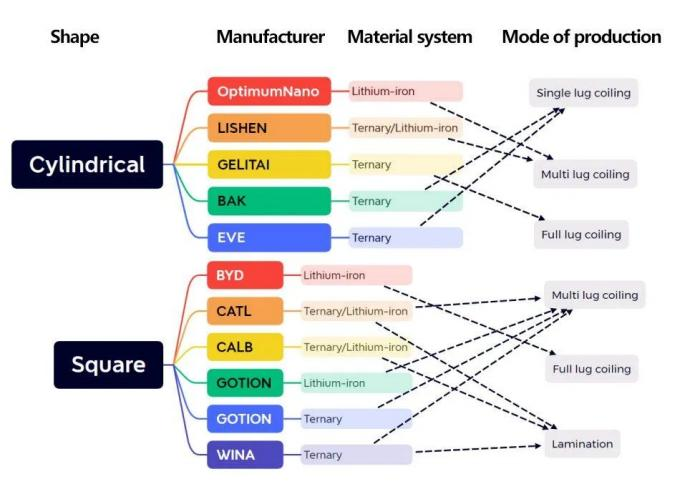
Lithium batteries can be classified by shape, casing and process.
Tritek The rolled cylindrical steel shell structure is used, mainly 18650 and 21700 batteries.
battery capacity
1I battery discharge capacity at room temperature (Ah) 1 (A) current reaches the end voltage.
Formula: C=It, that is, battery capacity (Ah) = current (A) x discharge time (h).
Battery capacity refers to the amount of power a battery can obtain or store.
The capacity is determined by the active material of the electrode and is mainly affected by the discharge rate and temperature (so strictly speaking, the battery capacity should specify the charging and discharging conditions).
The relationship between capacity and exhaust temperature:
Voltage
Refers to the potential difference (PD) between the positive and negative electrodes of the battery (the voltage is affected by battery power, temperature and other conditions).
How to choose the right voltage for your electric motorcycle battery
Open circuit voltage (OCV)
The voltage of a battery when it is not connected to an external circuit or external load. The open circuit voltage is related to the remaining energy of the battery, and the power display is based on this principle.
Closed circuit voltage (CCV)
It refers to the potential difference (PD) between the positive and negative electrodes of the battery under working conditions, that is, when current flows through the circuit.
Charging stage (SOC)
It is the remaining battery power ratio, which is equal to the remaining battery power/total battery power. SOC=0% means the battery is completely exhausted, and SOC=100% means the battery is fully charged.
SOC is calculated using the battery management system (BMS).
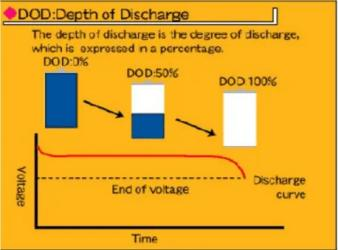
Depth of discharge (DOD)
It refers to the depth of discharge of the battery, that is, the percentage of battery discharge to the rated capacity of the battery. Contrary to SOC, DOD=100% means the battery is empty, and DOD=0% means the battery is fully charged. The relationship between DOD and SOC is as follows: DOD+SOC = 1.
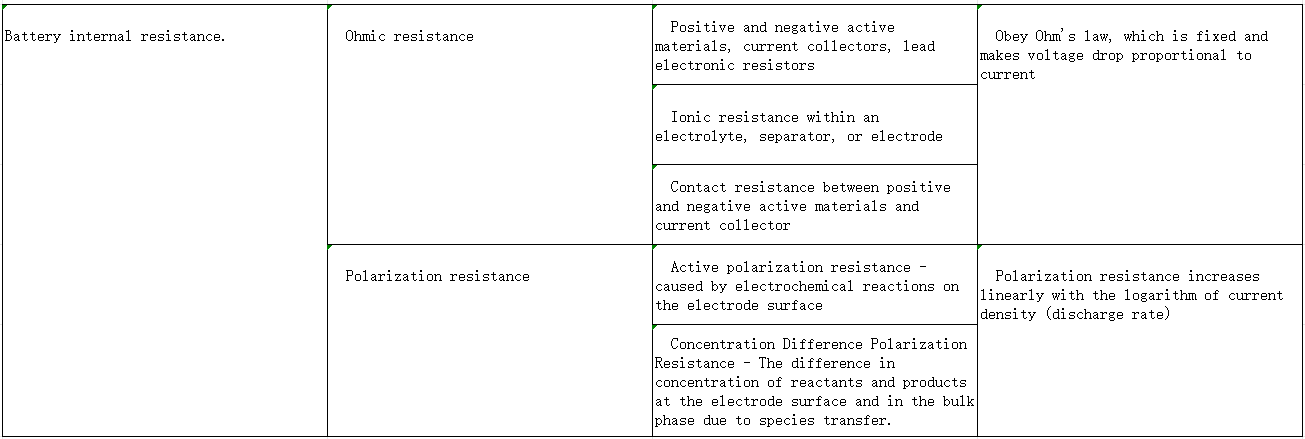
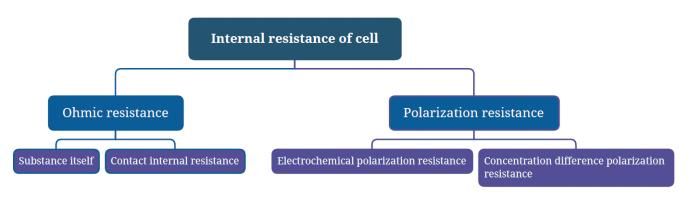
internal resistance
Refers to the resistance of the current flowing through the battery when the battery is working. Generally divided into AC (alternating current) internal resistance and DC (direct current) internal resistance. Normally, batteries with small internal resistance have strong discharge capabilities, while batteries with large internal resistance have weak discharge capabilities. The internal resistance of the battery will generate a large amount of Joule heat, which will increase the internal temperature of the battery, causing the battery discharge operating voltage to decrease and the discharge time to shorten, affecting battery performance and performance. Seriously affect the service life.
The temperature rises
The heat generated when current passes through a conductor Q=I2Rt
Discharge plateau time
The discharge plateau time refers to the time it takes for the battery to discharge to a certain voltage after it is fully charged. The discharge plateau is a characteristic of the battery discharge curve. The unit is minutes.
Charging constant current ratio
The ratio of constant current charging to the total constant current and constant voltage charging capacity. The higher the constant current ratio, the better the battery performance. The unit of constant current ratio is percentage (%).
cycle life
Battery cycle life refers to the number of charges and discharges experienced by a battery under a certain charge and discharge system when the battery capacity drops to a certain specified value.
Understanding lithium battery life: How many charge cycles can you expect?
Charge and discharge rate
The charge-discharge rate refers to the current required by the battery to discharge the rated capacity within a specified time. 1C is equal to the rated capacity of the battery, usually represented by the letter C.
self-discharge rate
Self-discharge rate, also known as charge retention rate, refers to the ratio of the reduced capacity to the initial capacity of a battery in an open circuit state after it is fully charged under certain conditions and stored for a certain period of time. The unit of self-discharge rate is percentage (%).
The smaller the self-discharge, the better, and the larger the charge retention, the better. Batteries with large self-discharge tend to experience a rapid voltage drop after being stored for a period of time. Mainly affected by manufacturing process, materials, storage conditions and other factors.
Charging efficiency
The degree to which a battery can store chemical energy is measured by converting the electrical energy consumed during charging. Mainly affected by battery technology, formula, ambient temperature, charging rate and other factors. Generally speaking, the higher the charging rate, the lower the charging efficiency. The lower the temperature, the lower the charging efficiency.
Discharge efficiency
Under certain discharge conditions, the ratio of the actual discharge amount of the battery from discharge to the end voltage and the rated capacity. Mainly affected by factors such as discharge rate, ambient temperature, and internal resistance. Generally speaking, the higher the discharge rate, the lower the discharge efficiency. The lower the temperature, the lower the discharge efficiency.
new energy
Formula: Energy (Wh) = Working voltage (V) × Working current (A) × Working time (h) = Voltage × Capacity
Specific energy (energy density)
The energy given by the battery per unit mass or unit volume is called mass specific energy or volume specific energy, also known as energy density.
Usually expressed in volume energy density (Wh/L) or mass energy density (Wh/kg). If the lithium battery weighs 143g, has a rated voltage of 3.2V, a capacity of 6500mAh, and an energy density of 145Wh/kg (3.2*6500/143).
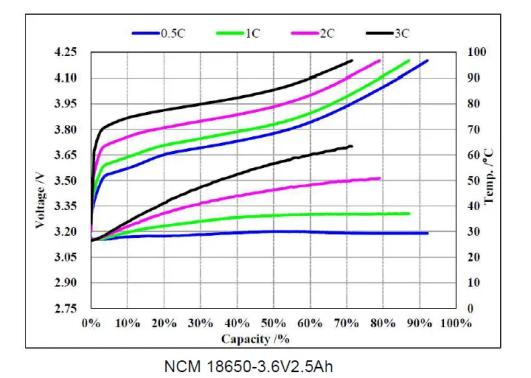
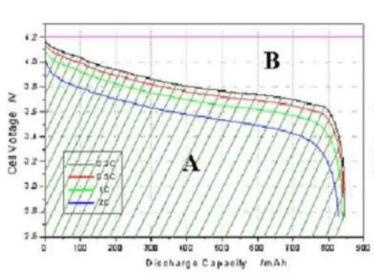
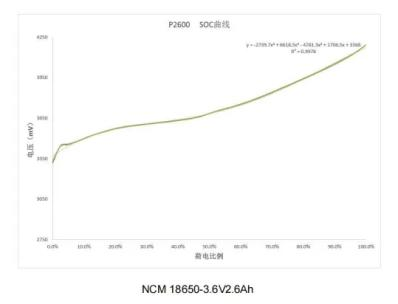
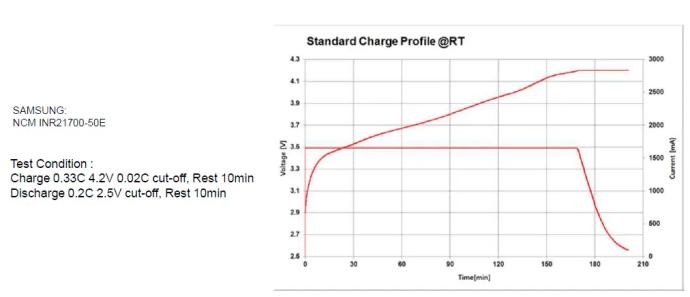
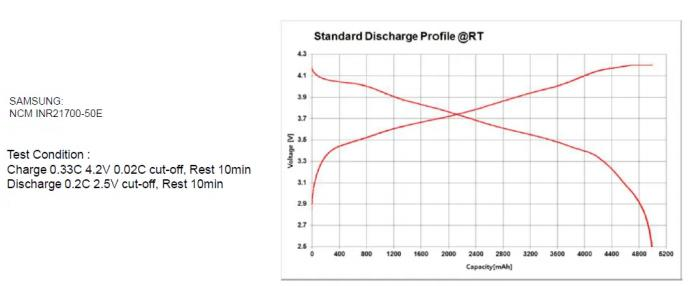
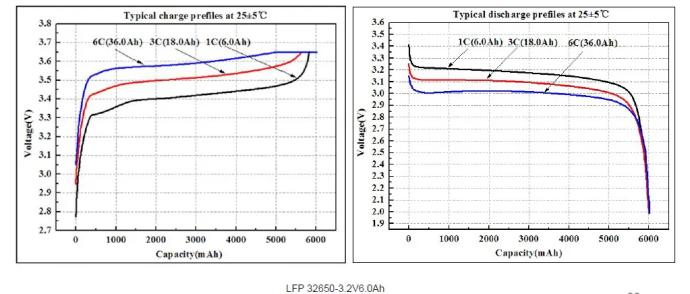
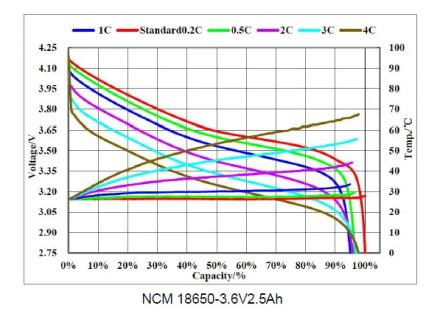
1.4 Lithium battery-charge and discharge curve
Charging curve
Discharge curve
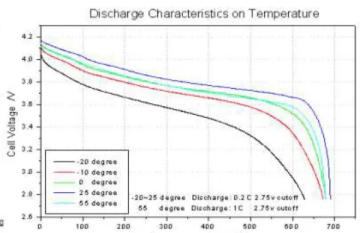
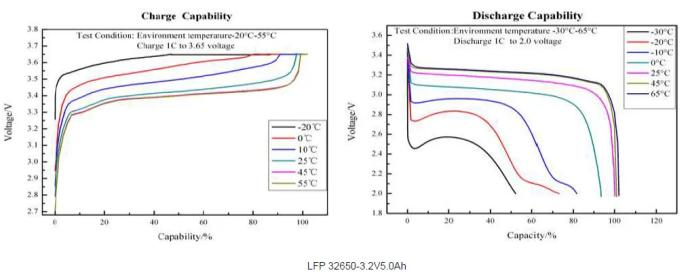
Lithium battery-high and low temperature performance
Lithium battery-high and low temperature performance
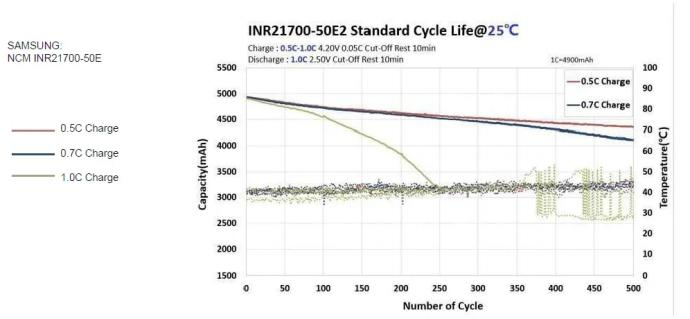
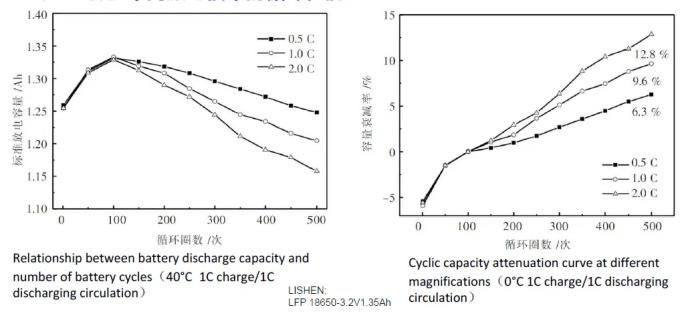
Lithium batteries – cycle performance at different charging rates
Lithium battery – cycle performance at different discharge rates
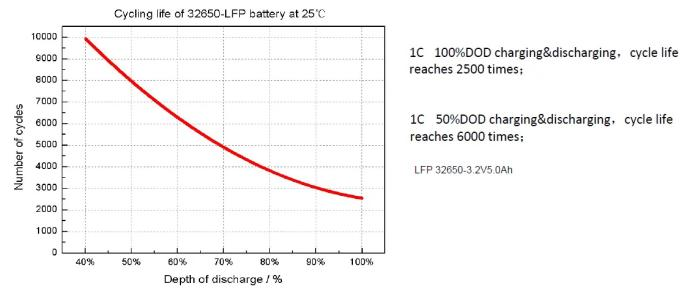
Lithium Batteries – Impact of DOD on Cycle Life
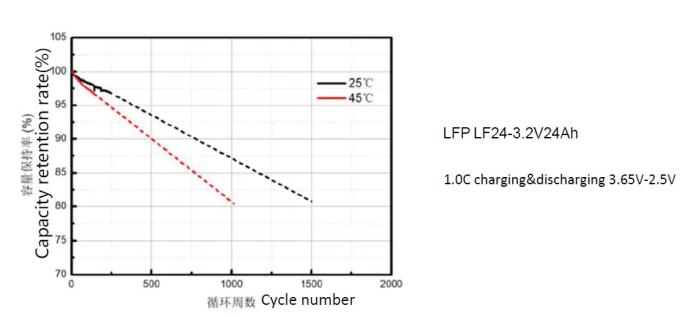
Lithium Batteries – Effect of Temperature on Cycle Life
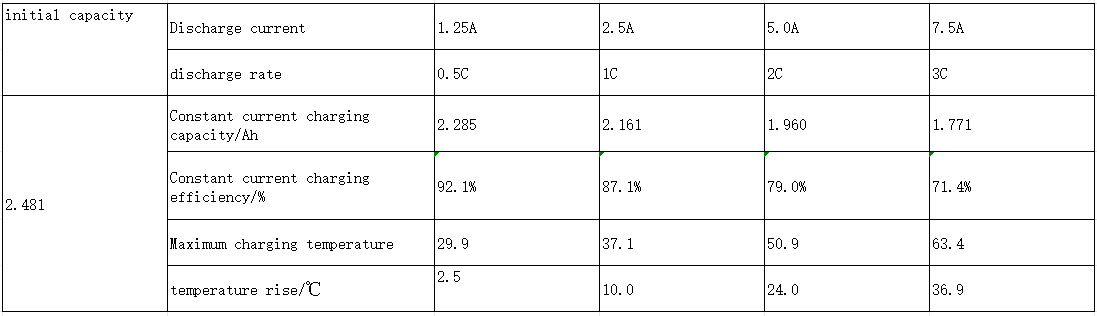
Lithium battery-normal temperature rate discharge, discharge efficiency, temperature rise
Normal temperature rate discharge
1. Charging: constant current and constant voltage, current 1.25A (0.5C), upper limit voltage 4.2V, cut-off current 0.05A (0.02C);
2. Let stand: 10min;
3. Discharge: constant current discharge with different sizes of current, lower limit voltage 2.75V;
4. Discharge efficiency = discharge capacity at each rate/0.5A (0.2C) discharge capacity;
Lithium battery – normal temperature rate charging, charging efficiency, temperature rise
Normal temperature rate charges
1. Discharge: current 0.52A (0.2C), constant current to 2.75V;
2. Let stand: 10min;
3. Charging: Use constant current and constant voltage charging of different sizes, the highest voltage is 4.2V;
4. Charging efficiency = constant current charging capacity at each rate/2.5A (1C) discharge capacity;
Lithium battery – principle of doubling charge and discharge temperature rise
The heat generated during the charging and discharging process of lithium batteries mainly consists of three parts:
Form: polarization heat, ohmic heat, reaction heat, reaction heat is endothermic reaction
responsible for;
The higher the charge-discharge ratio (current), the greater the polarization internal resistance and the greater the heat generation.
Lithium battery – matching battery PACK
“Eight Consistent” matching battery pack principles: consistent capacity, consistent internal resistance, consistent constant current ratio, consistent platform time, consistent self-discharge, consistent voltage, consistent charged charge, and consistent cycle.
Cellular material knowledge
Battery core material knowledge – positive electrode
Category of positive electrode: lithium iron phosphate, ternary NCM/NCA, lithium manganate, lithium cobalt oxide, lithium iron manganese phosphate.
Battery core material knowledge – negative electrode
Classification of negative active materials: artificial graphite, natural graphite, mesophase carbon beads, soft carbon, hard carbon, and carbon fiber.
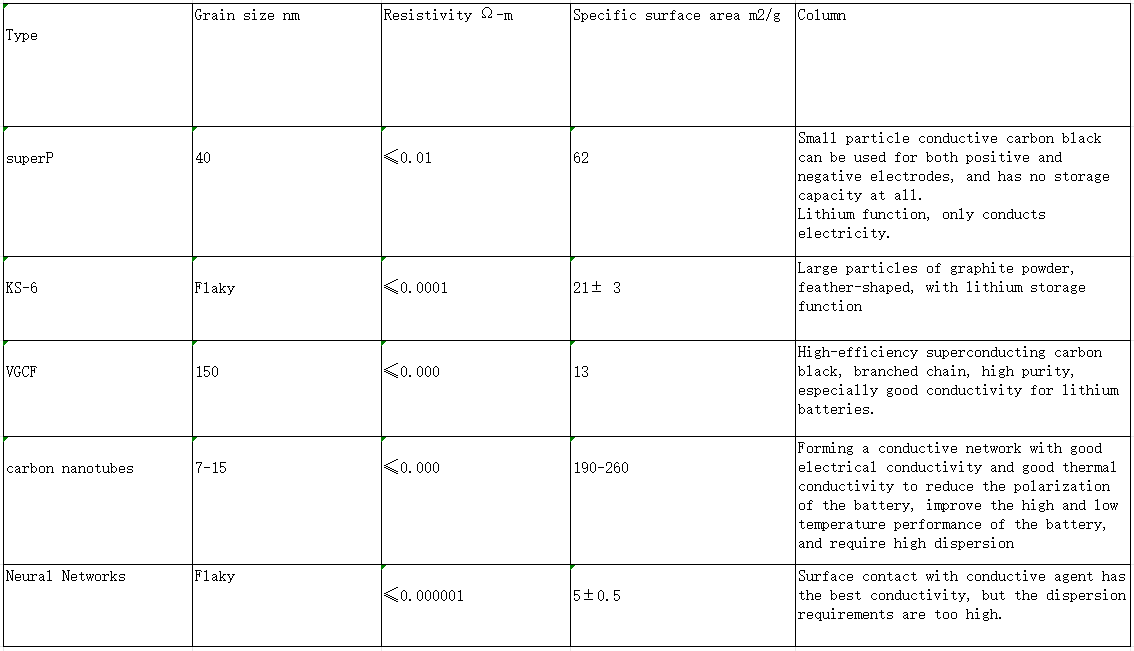
Battery core material knowledge – conductive agent
The conductive agent is to ensure that the electrode has good charge and discharge performance. When making electrode plates, a certain amount of conductive material is usually added to improve the charge and discharge efficiency of the electrode.
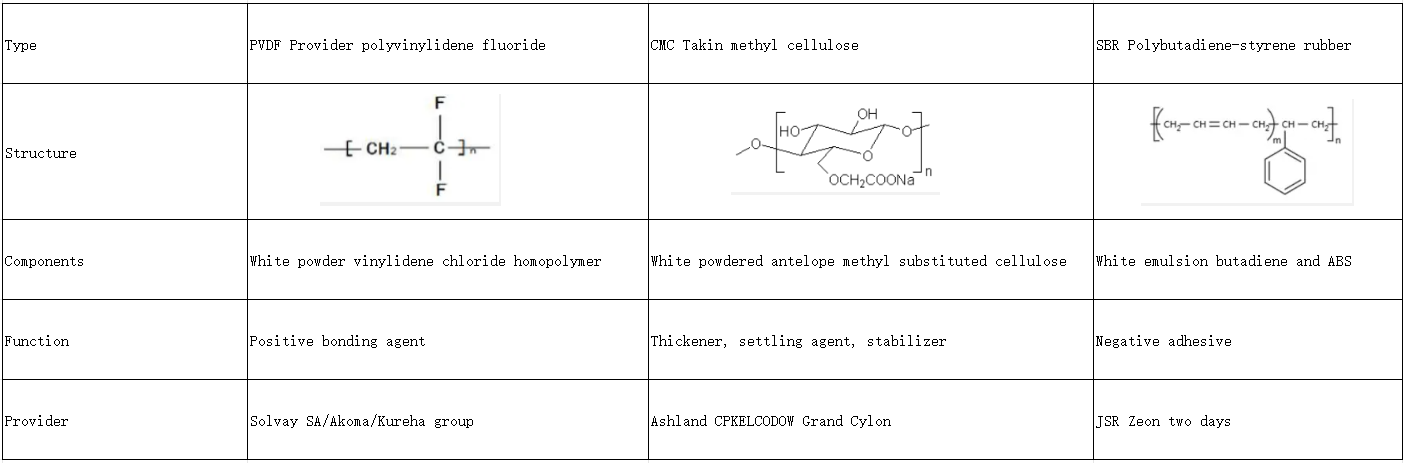
Battery material knowledge – adhesives
Battery core material knowledge – foil
The current collector refers to collecting the current generated by the active material of the battery to form a larger current output, with full contact with the active material, small internal resistance and good conductivity.
Aluminum foil – the positive electrode potential is high and the oxide film is very dense, which can prevent the current collector from oxidizing. The oxide film of copper foil is relatively loose. In order to prevent oxidation, low potential is better. Li and Cu are not easy to form lithium-embedded alloy at low potential.
Copper foil - The oxide film on the copper surface is a semiconductor and conducts electrons. The oxide film is too thick and the resistance is high; AL will be alloyed with LiAl at the low potential of the negative electrode, that is, Al will embed lithium in the negative electrode.
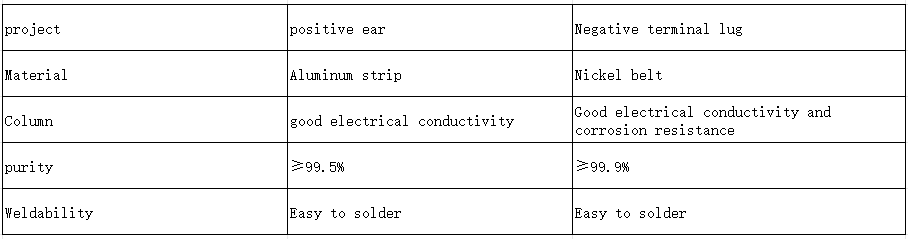
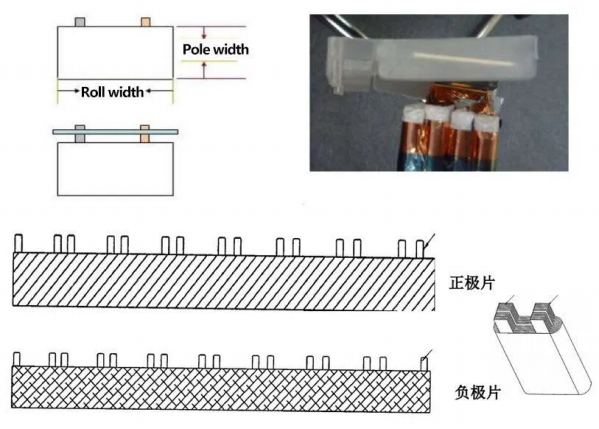
Battery core material knowledge——Lug
Lugs are the metal conductors that lead from the positive and negative terminals of the battery. Generally speaking, the tabs of the positive and negative poles of the battery are the contact points during charging and discharging.
Battery core material knowledge – diaphragm
Material: single layer PE (polyethylene) or three layers composite PP (polypropylene) + PE + PP
Function:
1.Separate the positive and negative terminals of the battery to prevent short circuit;
2.Adsorbs battery electrolyte to ensure high ionic conductivity;
3.Some also prevent harmful substances from transferring and reacting between electrodes;
4.It ensures that the battery stops responding when abnormality occurs and improves the safety performance of the battery.
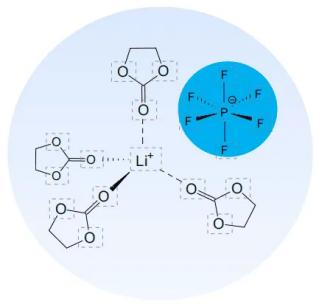
Battery material knowledge – electrolyte
The electrolyte plays a role in conducting ions between the positive and negative electrodes of the battery, and is a bridge connecting the positive and negative electrode materials. Only operate in a dry environment (such as a glove box with a humidity below 20 ppm).
1.Lithium salt: LiPF6
2.Solvent: EC, DMC, EMC
3.Additives: film-forming agent, anti-overcharge agent, flame retardant, stabilizer, etc.
Battery core material knowledge – steel shell
The main function of the battery steel shell is to provide a good electrochemical environment.
Battery steel case performance characteristics:
1.The material has good processing performance, high precision and high strength;
2.The battery surface has high hardness and has certain load-bearing performance.
3.Nickel plating: The battery has good corrosion resistance.
4.The bottom generally cannot be laser spot welded.
Battery material knowledge——Cap
The main function of the cap is to provide battery sealing function, provide a safety valve, and serve as a positive conductive terminal.
Bottle cap technical requirements:
1.The sealing ring is made of polyethylene propylene and will not deform at 135°C;
2.The surface is smooth, the wall thickness is uniform, and the appearance is not damaged;
3.The opening pressure of the bottle cap is 1.2±0.1Mpa, and the opening pressure is 1.8±0.1Mpa;
4.The small gasket will not deform or melt at 350°C;
5.Caps are generally not soldered at high temperatures.
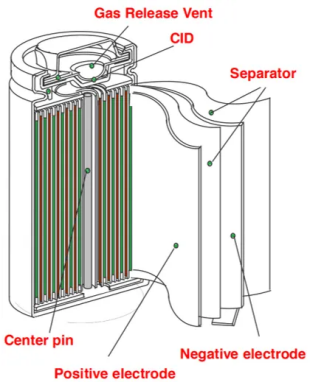
Battery core material knowledge – safety device
Some manufacturers’ cylindrical lithium iron phosphate batteries have built-in vents and safety valves (CID) to improve their safety protection functions. “Double protection” effectively solves overcharge, short circuit, collision, etc.
Vent device: When a chemical reaction occurs in the battery, the gas generated in the battery gathers in the vent through the vent hole and the upper and lower openings, which helps the gas in the battery to dissipate, ensures the air pressure balance of the battery cells, and avoids safety hazards.
Safety valve (CID): When the internal pressure reaches 1.2±0.1Mpa, the positive ear and cap are disconnected, and the chemical reaction in the battery is suspended. When the internal pressure reaches 1.8±0.1Mpa, the safety valve opens and the gas is discharged to avoid the risk of explosion.
Cell process knowledge
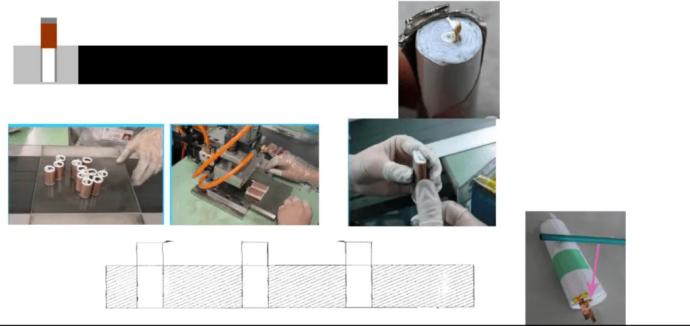
Cell process knowledge-process flow
The following is the process flow of the cell:
Cell structure knowledge
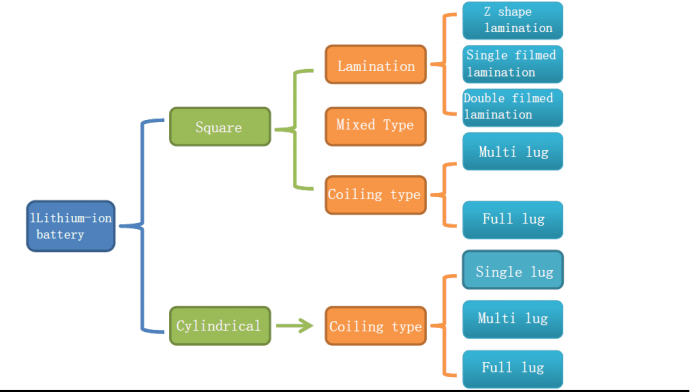
The following is a mind map of structural knowledge:
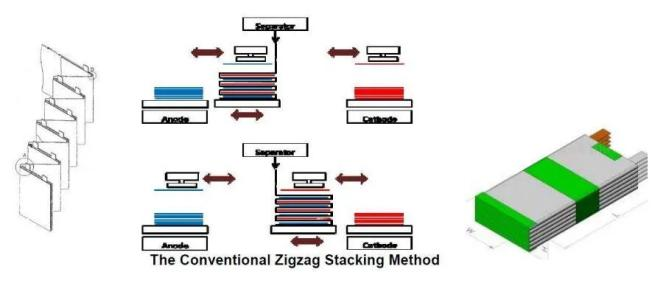
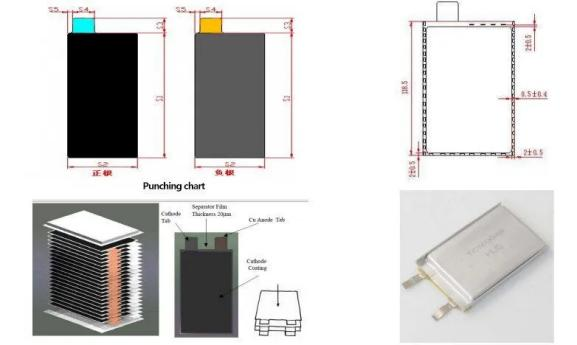
Battery core structure knowledge – square stack
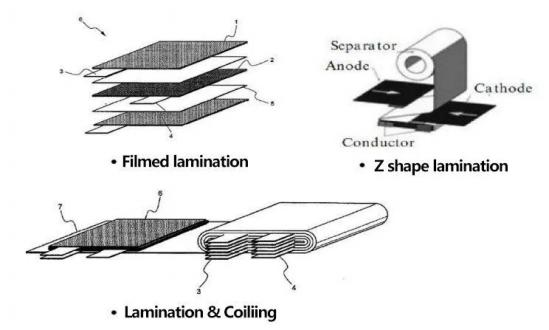
Battery core structure knowledge – square Z-shaped laminations
Battery core structure knowledge – square film lamination
Battery core structure knowledge – square multi-lug winding
Battery core structure knowledge – cylindrical single multi-lug winding
Battery core structure knowledge – cylindrical full-ear winding
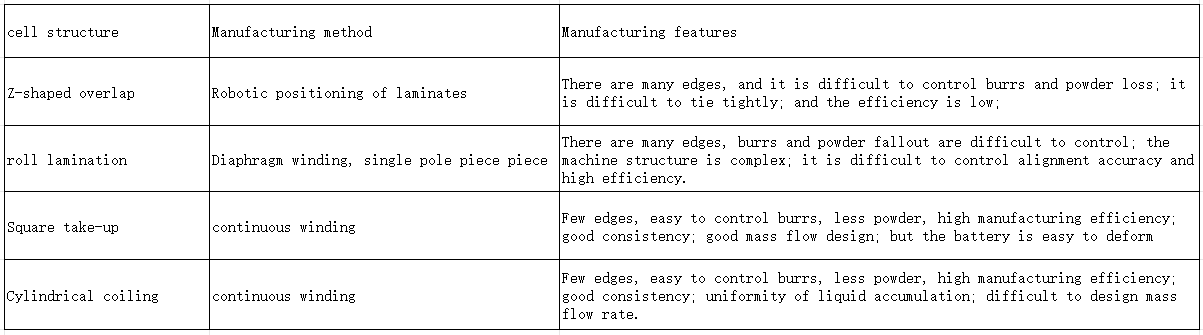
Cell structure knowledge – summary
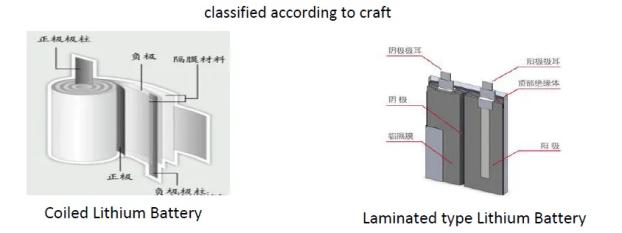
Summary: The advantage of rolling is that the process is easy to control, and the advantage of lamination is that the battery energy density is slightly higher and PACK is convenient.
summary:
Multiple lugs have many shortcomings, especially certain safety hazards; but when the capacity is small, it has the advantage of energy density.
The full lugg project is generally better, especially for the capacity battery, the full lug project should be the first choice.
Post time: Oct-14-2023





.png)